Gain total oversight over your document workflow
Here’s how eSign Documents takes your document quality management system (QMS) to the next level, from control and visibility to regulatory compliance.
Start
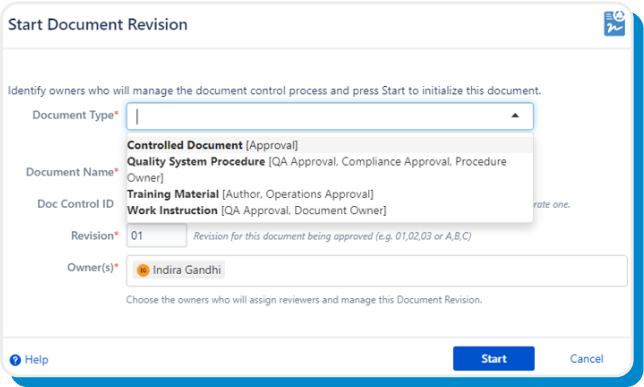
Tailor your workflow by configuring any number of document types and defining the approvals for each.
On Start, the user confirms the Document Type and eSign Documents will initialize the document and generate a unique document ID and the next revision.
Review
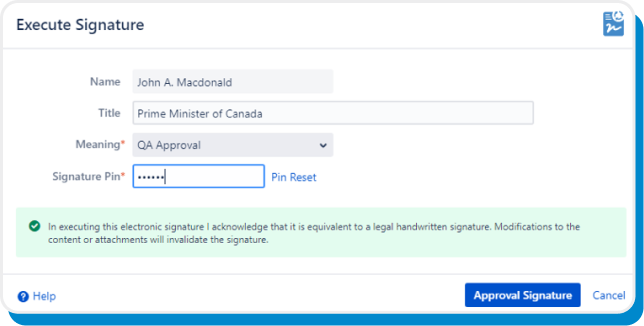
Prompted via email, reviewers approve or reject the latest version of the page with PIN-authenticated electronic signatures.
Once all reviewers have approved the page, the document is automatically finalized.
Release
The release workflow publishes an immutable snapshot of the approved document version to a designated space, while automatically obsoleting the previous revision, carrying forward training assignments to the latest version, and generating an Approval Record PDF.
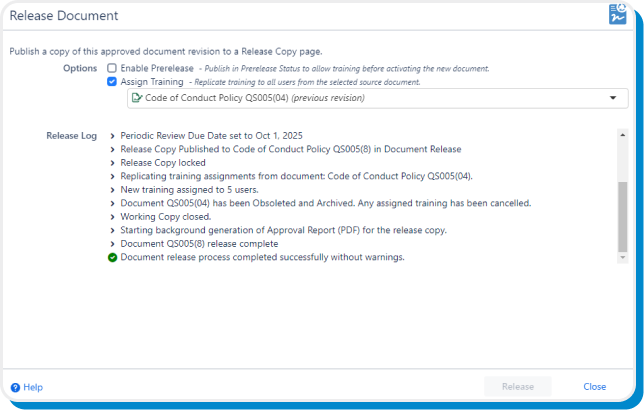
Training
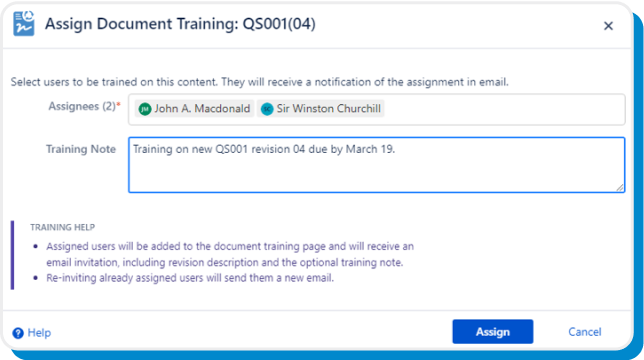
Use our built-in document training capability to assign training and due dates to users and groups, notify via email, and enable confirmation via e-signatures.
Generate printable training records anytime for audits and inspections.
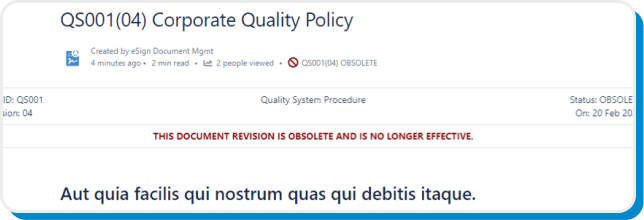
Obsolete
While new releases automatically and immediately obsolete previous versions, obsolete documents will also display a warning banner and can be archived to prevent users from unintentionally using end-of-life and outdated documents.
Integrated end-to-end document training capability
Products, documents, and revisions can be complex, but training your users on them doesn’t have to be. Assign, complete, and confirm document training, right within Confluence.
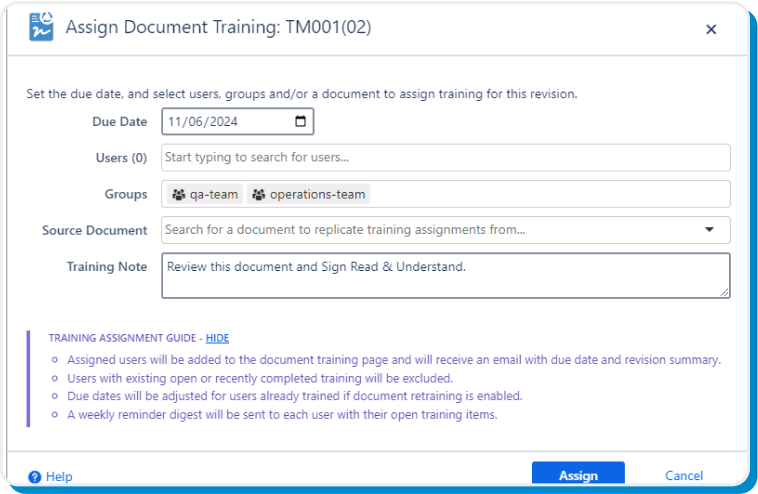
Assign training individually or in bulk, and set eSign Documents to automatically avoid recently trained users or those with an open training assignment. On top of that, set assignments to automatically carry forward to newly released revisions.
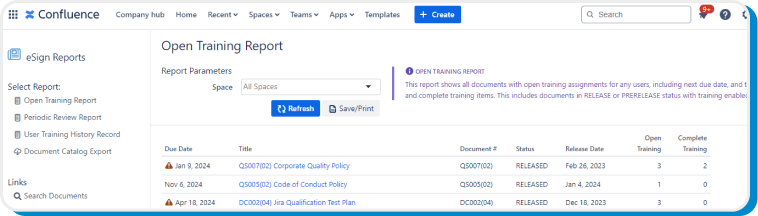
Use the Open Training Report feature to quickly view all documents with due or overdue training. Meanwhile, the app automatically sends weekly training reminder digests via email to users who have outstanding training assignments.
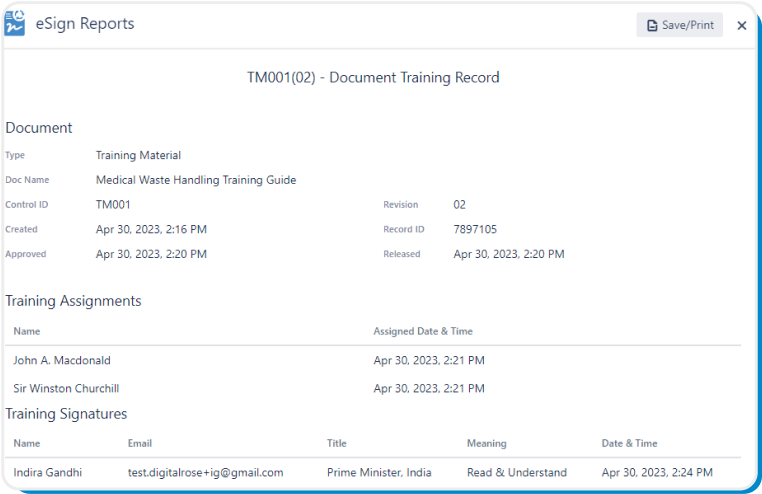
Users complete training with US FDA 21 CFR Part 11 compliant electronic signatures and as such, a printable Document Training Record with detailed signature data can be generated at any time for audits and inspections.
Audit and verification, without the frustration
Forget spending hours combing through Confluence docs, Jira issues, and chat histories. eSign Documents automatically creates a comprehensive trail detailing every document control activity for you to audit.
Don’t let unapproved edits and attachments slip through. eSign Documents automatically detects and flags content that’s modified after approval, and every approver must sign off on the latest changes before a document revision can be approved and released.
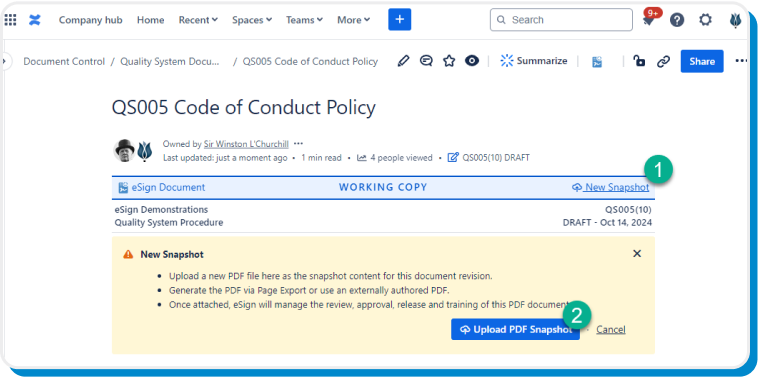
Capture and embed a static point-in-time snapshot of the page—including dynamic content macros—to give your teams pinpoint accurate information for reviews, approvals, and releases.
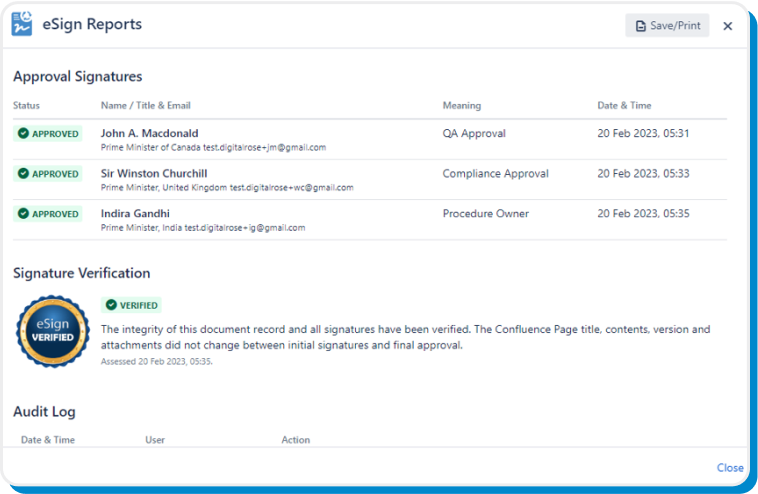
Easily audit every document control activity by accessing detailed document records at any time:
- Document Approval Record
- Document Training Record
- Document Audit Record

Strict compliance, airtight security

Data Residency
eSign Documents does not store any customer data—all signature data is stored securely and exclusively within your Atlassian Cloud infrastructure.

Regulatory Compliance
eSign Document’s comprehensive signature data archival and change-detection features are designed to help you thrive in regulatory compliant environments such as US FDA 21 CFR Part 11 and GDPR.

Secure Data Processing
eSign Document’s processing services are securely hosted by an ISO 27001 and SOC 2 compliant partners in a trusted region that you can choose.
Client Reviews
Digital Rose has been very responsive and supportive. Thank you for the constant improvements, support and the ever accepting attitude towards feature requests. We are very fortunate to receive such good support for a product of importance to our Jira implementation.
Digital Rose has been very responsive to requests. The addition of the bulk signatures feature adds significant efficiencies and meets all of our needs for electronic signatures.
The app works exactly as described and the support team is just stuning. We had a little problem with the PDF exports because the signature macro didn't came with, then I reported it and about 4 hours later they fixed it globally for all installations. That's how it should be done. :)
This is your sign to test-drive eSign Documents
Don’t just read about it. Take eSign Document Management & Training for a spin now.
DISCLAIMER
*Part 11 compliance requires procedural and administrative controls in addition to software such as eSign.
How Can We Help?
Have a question about an app? Need a new feature? Let’s chat.
Book a product demo
Book a live product demonstration with our team.
Book a technical Q&A
Book a time for our team to answer your questions.
Submit a support request
Need help with a specific issue? Click to submit a ticket.
View Documentation
Check the documentation for setup tips, feature info and more.